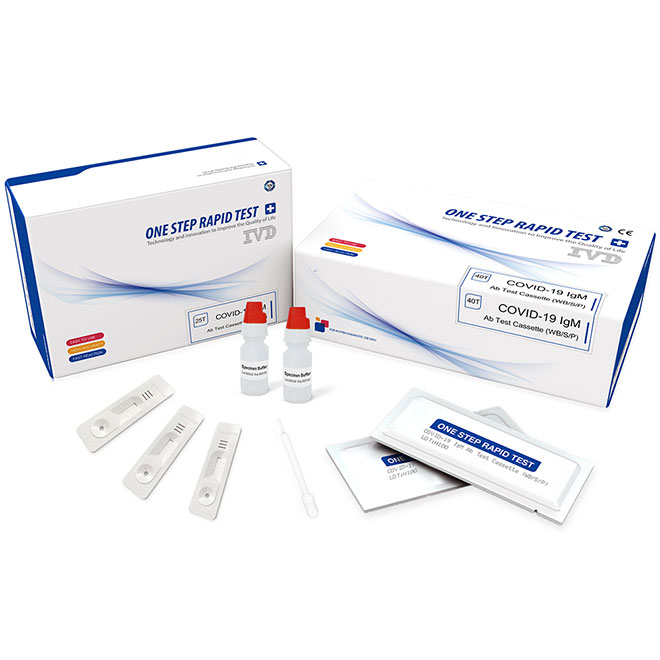
Quick Details
COVID-19 Anti- 2020-nCoV New Coronavirus
coronavirus test kit COVID-19 rapid test kit IgM/IgG test TUV
Novel Coronavirus COVID-19 IgG/IgM Diagnostic Rapid
Packaging & Delivery
| Packaging detail:Standard export package Delivery detail:within 7-10 workdays after receipt of payment |
Specifications
AMRPA68
COVID-19 IgM/IgG Antibody Rapid Test Kit
(Immunochromatography)
PRODUCT NAME
COVID-19 IgM/IgG Antibody Rapid Test Kit
(Immunochromatography)
INTENDED USE
The reagent is used to detect the Corona Virus-19 IgM/IgG Antibody in
serum/plasma/whole blood qualitatively.
TEST PRINCIPLE
This kit is based on the principle of gold label immunochromatographic test and uses capture method to detect the COVID-19 IgM/IgG antibody in the sample.
TEST PRINCIPLE
COVID-19 IgM
When the sample contains the COVID-19 IgM antibody, it forms a complex with the gold label antigen (COVID-19 recombinant antigen). The complex moves forward under the action of chromatography and combines with the coated antibody (Mouse anti-human IgM monoclonal antibody) at the T line to form a complex and develop color (T line), which is a positive result. When the sample does not contain the COVID-19 IgM antibody, no complex can be formed at the T line, and no red band appears, which is a negative result.
Regardless of whether the COVID-19 IgM antibody is contained in the sample, the gold label quality control antibody (rabbit IgG antibody) will bind with the coated antibody (goat anti-rabbit IgG antibody) at the C line to form a complex and develop color (C line).
COVID-19 IgG
When the sample contains the COVID-19 IgG antibody, it forms a complex with the gold label antigen (COVID-19 recombinant antigen). The complex moves forward under the action of chromatography and combines with the coated antibody (Mouse anti-human IgG monoclonal antibody) at the T line to form a complex and develop color (T line), which is a positive result. When the sample does not contain the COVID-19 IgG antibody, no complex can be formed at the T line, and no red band appears, which is a negative result.

Regardless of whether the COVID-19 IgG antibody is contained in the sample, the gold label quality control antibody (rabbit IgM antibody) will bind with the coated antibody (goat anti-rabbit IgG antibody) at the C line to form a complex and develop color (C line).
MAIN COMPONENTS
COVID-19 IgM: T-line coated with mouse anti-human IgM monoclonal antibody, gold label pad solid phase COVID-19 recombinant antigen, rabbit IgG antibody, C-line coated with goat anti-rabbit IgG antibody.
COVID-19 IgG: T-line coated with mouse anti-human IgG monoclonal antibody, gold label pad solid phase COVID-19 recombinant antigen, rabbit IgM antibody, C-line coated with goat anti-rabbit IgM antibody. Sample dilution: composed of 20 mM phosphate buffer solution (PBS)
STORAGE AND EXPIRY
Store as packaged in the sealed pouch at 4-30℃, avoid hot and sunshine, dry place, valid for 12 months. DO NOT FREEZE. Some protective measures should be taken in hot summer and cold winter to avoid high temperature or freeze thaw. Do not open the inner packaging until ready, it must be used in one hour if opened (Humidity≤60%, Temp: 20℃-30℃). Please use immediately when the humidity>60%.
SAMPLE REQUIREMENT
1. The reagent can be used for the serum, plasma and whole blood samples.
2. A serum / plasma / whole blood sample must be collected in a clean and dry container. EDTA, sodium citrate, heparin can be used as anticoagulants in plasma / whole blood samples. Detect immediately after collecting blood.
3.Serum and plasma samples may be stored at 2-8℃ for 3 days prior to assay. If testing is delayed more than 3 days, the sample should be frozen (-20℃ or colder). Repeat freeze and thaw for no more than 3 times. Whole blood samples with anticoagulant can be stored at 2-8℃ for 3 days, and should not be frozen; whole blood samples without anticoagulant should be used immediately (if the sample has agglutination, it can be detected by serum) .
TEST METHODS
Instructions must be read entirely before taking the test. Allow the test device controls to equilibrate to room temperature for 30 minutes (20℃-30℃) prior to testing. Do not open the inner packaging until ready, it must be used in one hour if opened (Humidity≤60%, Temp: 20℃-30℃). Please use immediately when the humidity>60%.
For Serum/Plasma
1. Remove the test device from the sealed pouch, place it on a clean and level surface with the sample well up.
2. Add one (1) full drop of serum or plasma (10μl) vertically into the sample well of IgM and IgG separately.
3. Add two (2) drops (80-100μl) of sample buffer into the sample well of IgM and IgG separately.
4. Observe the test results immediately within 15~20 minutes, the result is invalid over 20 minutes
COVID-19 IgG
Analysis of coincidence rate of COVID-19 IgG Ab rapid test and nucleic acid reagent in serum samples:
Positive coincidence rate=46 / (46+4) × 100% = 92%,
Negative coincidence rate=291 / (9+291) × 100% = 97%,
Total coincidence rate=(46+291) / (46+4+9+291) × 100% = 96.3%.
COVID-19 IgM
Analysis of coincidence rate of COVID-19 IgM Ab rapid test and nucleic acid
reagent in serum samples:
Positive coincidence rate=41 / (41+9) × 100% = 82%,
Negative coincidence rate=282 / (18+282) × 100% = 94%,
Total coincidence rate=(41+282) / (41+9+18+282) × 100% = 92.3%
ATTENTIONS
1. For IN VITRO diagnostic use only.
2. Reagents should be used as soon as possible after opened. This reagent cannot be reused for disposable.
3. The test device should remain in the sealed pouches until use. If sealing problem happens, do not test. Don’t use after the expiration date.
4.All specimens and reagents should be considered potentially hazardous and handled in the same manner as an infectious agent after use.