High Quality Antigen Rapid Test Kit – COVID-19 Saliva Antigen test kit AMDNA09 – Amain Detail:
Quick Details
Incubation Period: 1~14 days, mostly 3~7 days
The minimum detectable limit varies with methodology and sensitivity of test
Antibody Window Period : 5-10 days after onset of symptoms
IgG antibody test can be referred as one of discharge criteria for recovering COVID-19 patients
Packaging & Delivery
| Packaging detail:Standard export package Delivery detail:within 7-10 workdays after receipt of payment |
Specifications
COVID-19 Saliva Antigen test kit AMDNA09
No professional training needed for sampling
No more discomfort during sampling
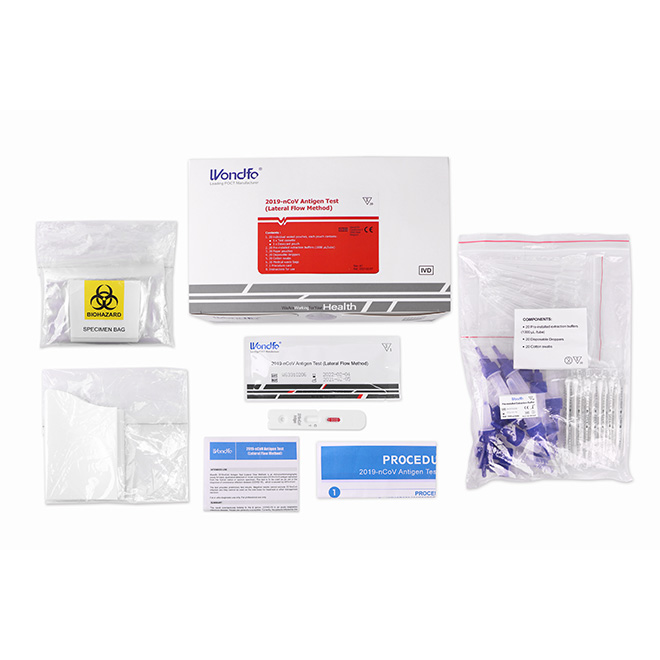
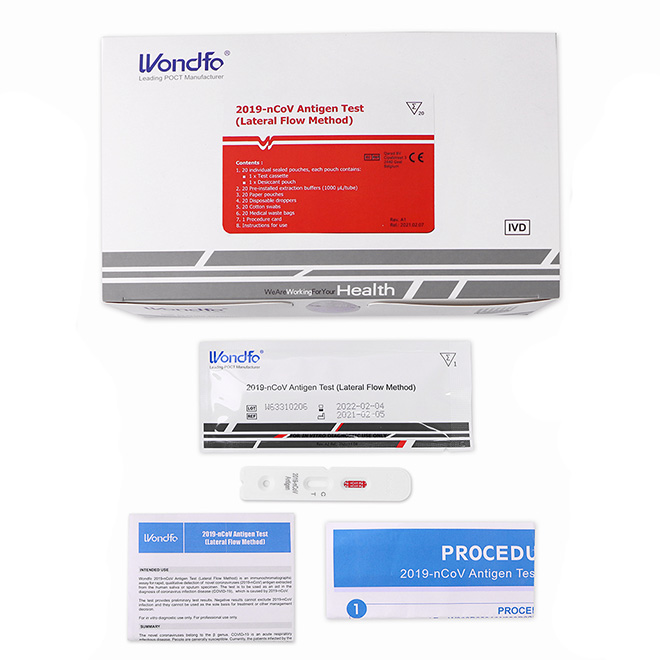
COVID-19 Saliva Antigen test kit AMDNA09
Incubation Period: 1~14 days, mostly 3~7 days
The minimum detectable limit varies with methodology and sensitivity of test
Antibody Window Period : 5-10 days after onset of symptoms
IgG antibody test can be referred as one of discharge criteria for recovering COVID-19 patients
Saliva/Sputum antigen test can be applied for initial screening of COVID-19, the positive result indicates the active infection.
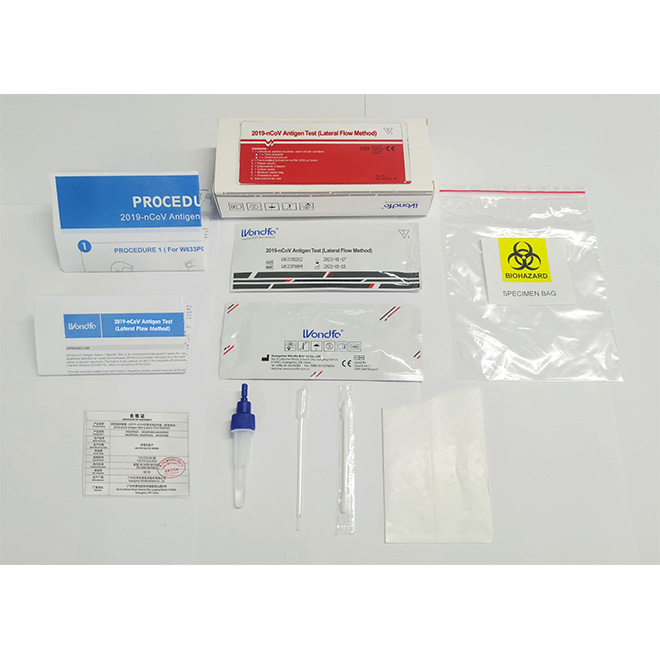
COVID-19 Saliva Antigen test kit AMDNA09 Material
Trockene Tupfer wurden in PBS aufgenommen, feuchte Tupfer waren bereits in Transportmedium unterschiedlicher Zusammensetzung. Pools sind zufällige Mischungen aus bis zu 10 Proben vergleichbarer CT Werte, die 1:10 in negativen Proben in PBS verdünnt wurden. Die CT Werte eines Pools wurden mit verschiedenen PCR Assays bestimmt und die mutmassliche Anzahl an RNA-Kopien mit Hilfe des INSTAND Standards berechnet. Bei den verwendeten PCRs entspricht ein CT Wert von 25 etwa 106 RNA Kopien / mL. Es wurden jeweils 18 Proben mit CT<25, 23 Proben mit CT zwischen 25 und 30 und 9 Proben mit CT>30 analysiert. Vermehrung des Virus in Zellkultur wurde als mögliches Korrelat für Infektiosität als weiteres Merkmal der Proben bestimmt.
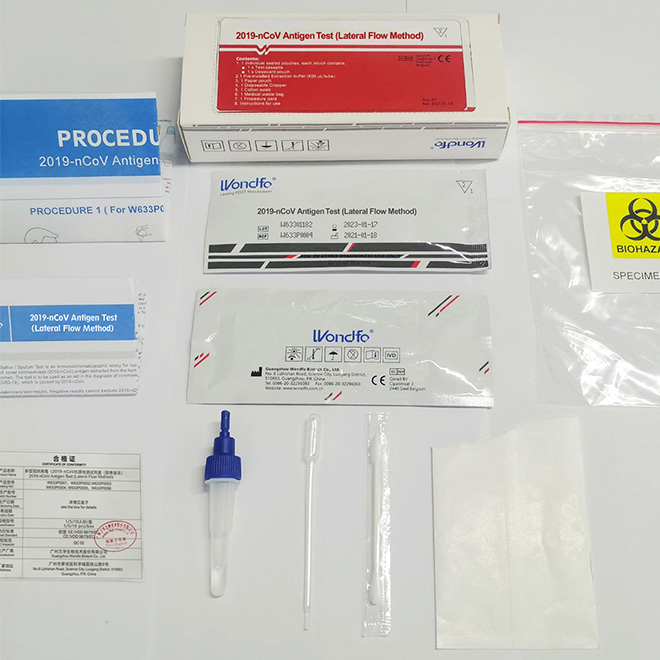
Product detail pictures:




Related Product Guide:
Our company puts emphasis on the management, the introduction of talented personnel, and the construction of staff building, trying hard to improve the quality and liability consciousness of staff members. Our company successfully attained IS9001 Certification and European CE Certification of High Quality Antigen Rapid Test Kit – COVID-19 Saliva Antigen test kit AMDNA09 – Amain, The product will supply to all over the world, such as: Japan , Pakistan , Montpellier , Products have been exported to Asia, Mid-east,European and Germany market. Our company has constantly been able to update the products performance and safety to meet the markets and strive to be top A on stable quality and sincere service. If you have the honor to do business with our company. we will definitely do our very best to support your business in China.
This is a very professional wholesaler, we always come to their company for procurement, good quality and cheap.